- Clairia AI
- Solutions
- Who We Help
- Resources
- Why Case IQ
With a set of tools developed specifically for life sciences companies, Case IQ provides a robust compliance workflow, compliance monitoring and third-party risk management solution to prevent and detect risk in activities with HCPs, HCOs, distributors and other third parties.

Track engagements with HCP/Os from initial selection, needs assessment, profiling and contracting through to proof of performance. Use data analytics to monitor all financial transactions related to those HCPs and HCOs.


Provide company users with the ability to profile HCPs and automate the FMV calculation and exception process, without needing to consult separate rate card tools or spreadsheets. Manage those FMV rates over time effortlessly and integrate profiled rates into HCP engagement workflows automatically.
Our compliance monitoring software allows teams to detect kickbacks, fraud and corruption in HCP, HCO and other third-party transactions. Connecting data from existing enterprise systems and using the power of algorithmic analysis, our compliance monitoring software enables early detection of high-risk transactions with HCP/Os or other third parties, such as vendors and distributors.
Our monitoring platform also ingests all historical CMS Sunshine Act Open Payments data across all companies to provide analytics across the industry on disclosed payments.


Case IQ helps life sciences companies reduce the regulatory and reputational risks involved with providing external funding, in the form of grants, sponsorship and donations. It provides a centralized record of both the rationale and approvals for such activity, a vital requirement for compliance risk detection and mitigation.
Our team of veteran in-house compliance and audit leaders applied years of in-house learning to create the most user-friendly life sciences compliance software on the market today.
Roll out a truly global and intuitive compliance platform to your employees, risk specialists, HCP/Os and third parties, with Case IQ.



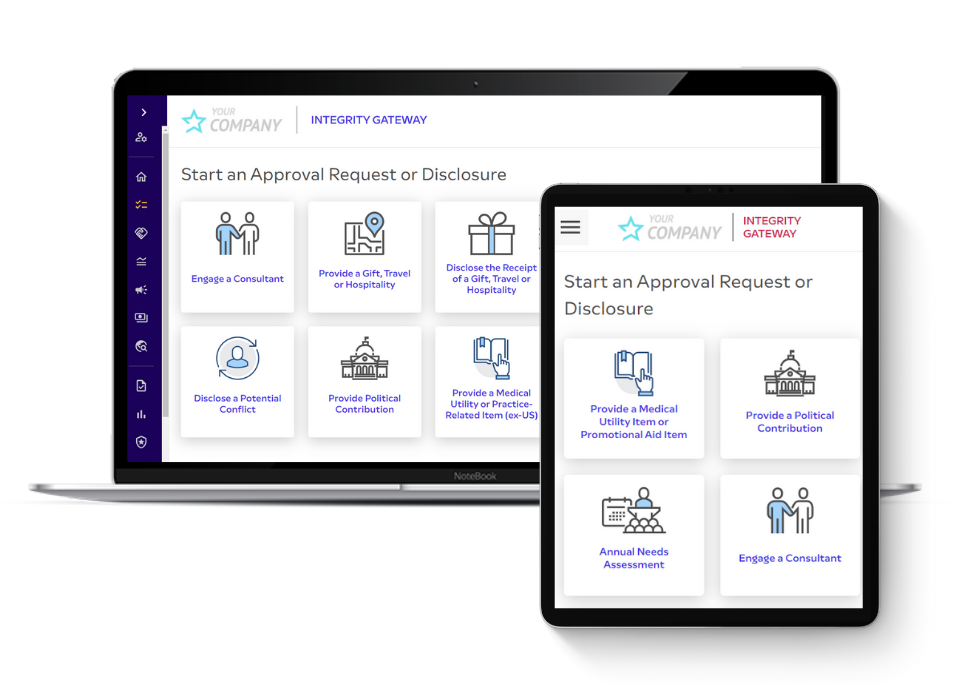
Our acclaimed software adapts easily to your organization’s workflow, so you don’t miss a beat. As soon as you’re up and running, you’ll find day-to-day tasks becoming more intuitive and efficient. You’ll also be able to spot patterns and trends, helping you mitigate organizational risks.
